Is the FDA “doubling down” on a failed strategy?
"We’re using a poor man’s immune correlates of protection"
Last Thursday, the FDA convened its Vaccines and Related Biological Products Advisory Committee (VRBPAC) to discuss the future of covid vaccines.
The panel voted 21 to 0 in favor of moving towards a more simplified vaccine schedule - an annual shot which would be updated as new variants emerge - much like the annual flu shot.
Despite the unanimous vote, VRBPAC members did raise concerns about knowledge gaps and questioned the need to boost everyone, as well as the futility of chasing rapidly mutating viruses.
But it all fizzled out quickly, and the FDA promised to reconvene in May or June to discuss the data further.
That said, I had some interesting observations of my own.
Still no correlate of protection
We are three years into the pandemic, and the FDA has still not established a “correlate of protection” for the vaccines.
Eight COVID-19 vaccine emergency use authorizations (EUAs)* have been granted, based on their ability to induce “neutralizing antibodies,” a surrogate marker of protection.
The idea is, the more antibodies you produce, the better you are protected.
Except, neutralizing antibodies do not predict the degree to which someone is protected from infection… and the FDA knows it.
Ofer Levy, VRBPAC member and Professor of Pediatrics at Boston Children's Hospital first voiced his concern at the April 6, 2022 meeting.
“We’re at risk of doubling down on a failed strategy,” said Levy as the committee discussed a framework for offering annual covid shots for Americans.
“Where is the federal effort to coordinate all of that to develop a public repository around the correlate of protection, and to make sure we have the best available data for the immunogenicity when we make those decisions?”
The FDA’s top vaccine official, Peter Marks, agreed with Levy.
“There is not a clear, perfect, immune correlate of protection” admitted Marks, “We’re using poor man’s immune correlates of protection here -- or poor person’s immune correlates of protection with antibody levels.”
In Dec 2022, Peter Marks reiterated these concerns in an article published in JAMA. He and his co-authors wrote:
“Therefore, unless correlates of protection that are strongly associated with duration of protection against COVID-19 can be identified, it is likely that rather than relying on immunobridging to infer vaccine effectiveness, large randomized clinical trials similar to the initial trials of the currently authorized or licensed vaccines for COVID-19 will be required to ascertain the effectiveness of these new vaccines.”
But fast forward to this latest meeting, and it becomes clear that we’re all still in the dark.
We have no correlate of protection, the FDA is relying heavily on real world studies (confounded data) and the agency still has not demanded any randomized controlled trials to show the bivalent booster can reduce severe disease or hospitalizations.
It’s no wonder doctors are coming out in droves, refusing to have any more COVID shots until the FDA demands better studies.
“I don't think we can say with credibility what the objective benefits are for someone like me to take an additional dose, nor what the rate of any rare but important side effects would be,” tweeted Todd Lee, a physician certified in Infectious Diseases and General Internal Medicine in Quebec, Canada.
Similarly, Vinay Prasad, haematologist-oncologist at the University of California San Francisco vowed not to take any more shots until there were data from randomized controlled trials.
“I took at least 1 dose against my will. It was unethical and scientifically bankrupt. I am not done with that error. No more,” he tweeted.
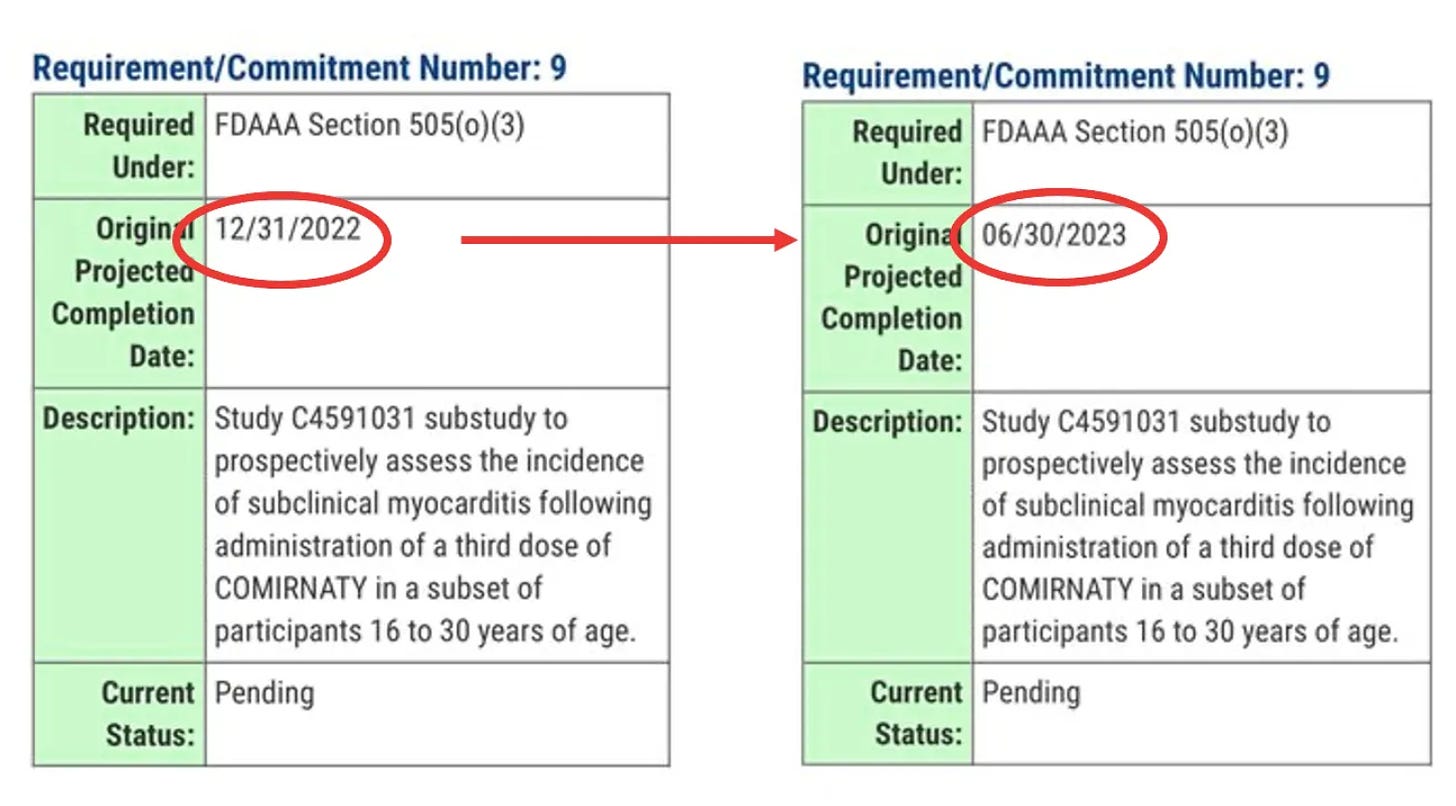
No update on subclinical myocarditis
As part of its post-marketing requirements, Pfizer is legally obligated to conduct a study involving people aged 16 to 30 to look at rates of subclinical myocarditis (i.e. underlying damage to the heart muscle without causing symptoms).
The final report was due 31 Dec 2022, but that deadline lapsed, and the FDA said nothing. There was no mention of the study, neither in the briefing notes ahead of the VRBPAC meeting, or during the meeting.
I asked the FDA directly for access to Pfizer’s study, but the agency said in an email, “You may submit a FOIA request for this information, or if you would like it more quickly, you can reach out to the manufacturer directly.”
Pfizer did not respond to my request, and the FDA refused to confirm whether it had even received Pfizer’s study, before abruptly ending our communication.
Jessica Adams, an expert in drug regulatory affairs pointed out on twitter that the FDA had quietly changed the due date for the study from 31 Dec 2022 to 30 June 2023.
So, now as it stands, millions of young people will receive boosters, mandated or not, without knowing if the vaccine is causing subclinical myocarditis.
FDA still working from home
Finally, the meeting was again held online because the majority of FDA employees are still working from home.
Since all federal employees have been mandated to take the COVID-19 vaccine to “protect themselves and those around them,” why aren’t they conducting face-to-face meetings?
“FDA leaders are in a bubble. How much longer will the FDA (18,000-employees) continue to work remotely? It’s mid-day on a weekday and the parking lot is essentially empty” tweeted Marty Makary, surgeon and public policy researcher at Johns Hopkins University.
“The FDA was telling the rest of America to get vaccinated, mask up and go back to work, but the FDA mysteriously did not follow its own advice,” said David Gortler, drug safety expert and former senior advisor to the FDA commissioner.
Well, it’s as though the FDA heard the cries.
Today, the FDA announced that “staff will be transitioning to a hybrid workplace.”. This transition will enable face-to-face formal meetings between FDA and industry to resume within weeks.
*FDA issued eight EUAs based on neutralizing antibodies (immunobridging studies) - an unproven correlate of protection.
Pfizer EUA - 6 months to 4 year olds
Pfizer EUA - 5 to 11 year olds
Pfizer EUA - 12 to 15 year olds
Pfizer EUA booster #1
Pfizer EUA booster #2
Moderna EUA - 6 months to 17 year olds
Moderna EUA - booster #1
Moderna EUA - booster #2




Thanks Good Sir.
At this point, I’d no sooner take any vaccine than would USS Indianapolis Shark Week-educated Robert Shaw (Quint) put on a life jacket again in “Jaws”.
Speaking of movies: Mel Gibson in “Braveheart”: “It’s well beyond rage”.
The Rebel Alliance grows.
How can the FDA be objective and unbiased when they are greatly influenced by the mRNA supporters and producers who see
These injections to be the future of the pharmaceutical industry that continues to embrace allopathic medicine as the primary solution to disease states. All you have to do is follow the money that is being invested by somewhere around 100 drug manufacturers to develop more nano drug injections.