Whatever you may currently think about the SARS-CoV-2 vaccines, it is a fact that more than 5.41 billion people worldwide have received a dose of some type of COVID-19 vaccine, equal to about 70.5 percent of the world population. In the United States as of October 17, 2022, 494.74 million “initial protocol doses” of SARS-CoV-2 vaccine have been administered, together with 138.16 million “booster” doses. 265.59 million US residents have received at least one dose, and 226.59 million have completed the initial vaccination protocol (see this link), out of a total population of 335.49 million (67.5%). In terms of the logistics of development, manufacturing and deployment of a novel injectable biologic product, this is undeniably a major achievement. Of the SARS-CoV-2 mRNA vaccine doses administered in the United States as of October 19, 2022, 375.64 million were manufactured by Pfizer/Bio-N-Tech, and 237.61 doses by Moderna, for a total of 613.25 million mRNA vaccine doses administered. In the European Union, the corresponding numbers are 641.89M doses of Pfizer/Bio-N-Tech and 153.16M doses of Moderna for an EU total of 795.05M mRNA vaccine doses, and a grand total of 1 Billion, 408.3 million doses of mRNA vaccines in these two regions. All this involves a novel technology, product and large scale manufacturing process which was created, passed non-clinical and clinical development and was massively manufactured, distributed and globally deployed in less than three years.
At a meeting of the Special Committee of the European Union Parliament held on 11 October 2022 to discuss the findings regarding COVID-19 pandemic and recommendations for the future, a Pfizer executive confirmed that the vaccine had never been tested for its ability to prevent the transmission of SARS-CoV-2 virus before being put on the market. Data emerging since the introduction of the vaccine indicates that it is in fact unable to do so, thereby refuting the claim that the COVID-19 Passports provide any guarantee of protection. In other words, although governments throughout the world employed a wide range of propaganda and censorship methods to promote these products as both safe and effective at stopping the spread of SARS-CoV-2 infection, there were no studies performed prior to this distribution which even tested how well the products would prevent the spread of COVID-19 disease. It is not an exaggeration to state that this massive deployment has been the largest clinical experiment performed on human beings in the history of the world.
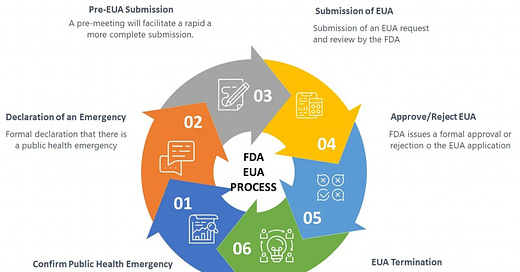
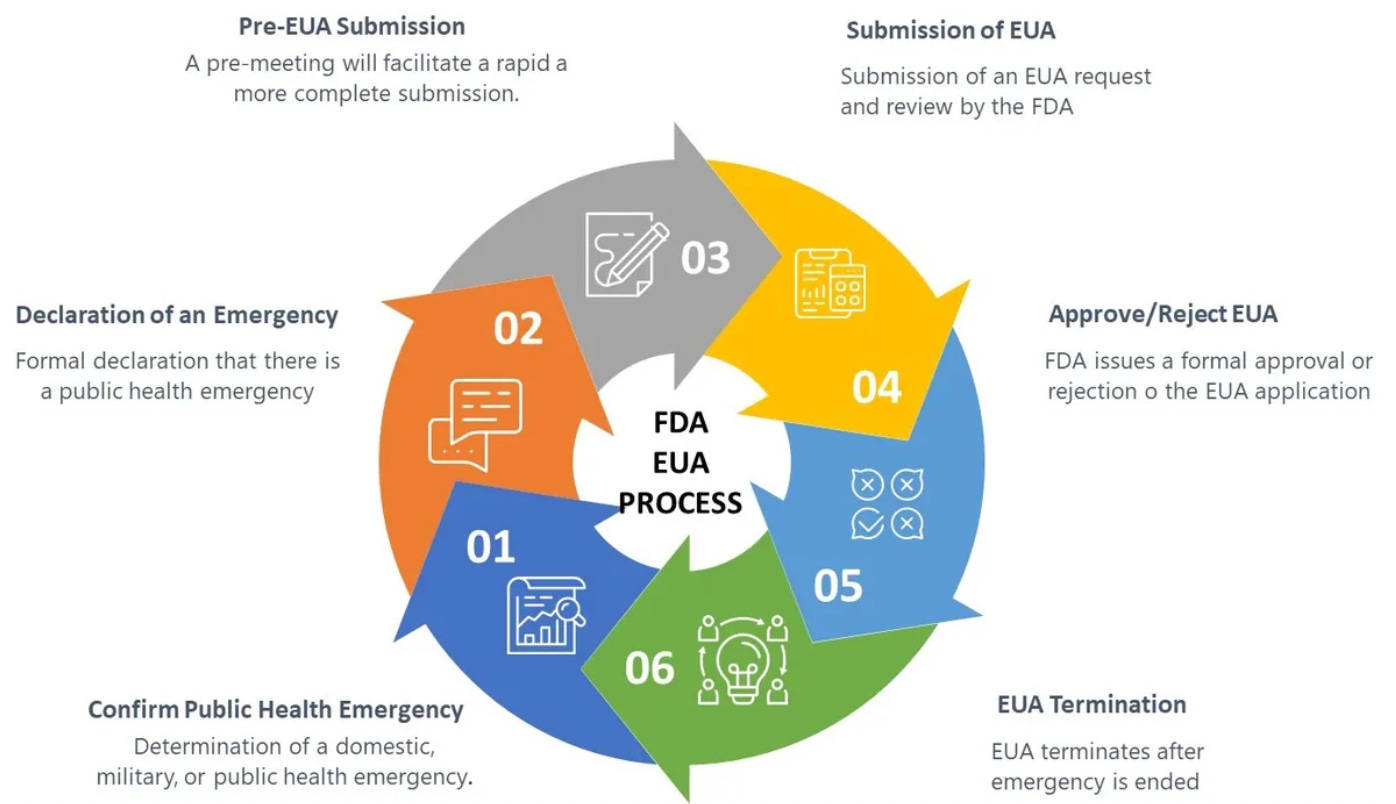
All of the mRNA vaccine doses administered in the United States (to both citizens and military personnel) have been provided under “Emergency Use Authorization” (EUA), which is to say that although the FDA has licensed the Pfizer/Bio-N-Tech and Moderna vaccines for some age cohorts, the firms have elected to not manufacture, distribute, or market these licensed products in the United States. The reason for this is not clear, but appears to relate to both liability issues as well as conditions placed by the FDA involving additional clinical studies, safety monitoring (pharmacovigilance) and product disclosures once the products begin to be marketed.
From the standpoint of the vaccine manufacturers, EUA is a preferred pathway for marketing their products. A single purchaser (the US Government) provides complete liability indemnification, a guaranteed market with very little oversight, and manages both the distribution and marketing. In the case of all unlicensed products, the manufacturers are prohibited from marketing them, but under EUA the US Government has been doing this for them, and has been acting in coordination with corporate media, social media, and large technology firms to suppress any discussion of risks or limitations of the products. From the standpoint of the vaccine manufacturers, this is all profit and no risk; a perfect business model. Why would they ever want to consider taking up the burden of actually producing and marketing the licensed version of these products?
EUA is a process defined by US federal law (21 U.S. Code § 360bbb–3 - Authorization for medical products for use in emergencies) which in the case of these mRNA-based products involves biological products which are not approved, licensed, or cleared for commercial distribution. Specifically, the statute authorizes “the introduction into interstate commerce, during the effective period of a declaration under subsection (b), of a drug, device, or biological product intended for use in an actual or potential emergency.” Continued “Emergency Use Authorization” of these vaccines requires “a determination by the Secretary of Homeland Security that there is a domestic emergency, or a significant potential for a domestic emergency, involving a heightened risk of attack with a biological, chemical, radiological, or nuclear agent or agents”. Once the domestic emergency has passed (ergo “a determination by the Secretary, in consultation as appropriate with the Secretary of Homeland Security or the Secretary of Defense, that the circumstances described in paragraph (1) have ceased to exist”), “A declaration under this subsection shall terminate”. In other words, when there is no longer an emergency, the “Emergency Use Authorization” for the product will cease, and the vaccine products will return to their status as not approved, licensed, or cleared for commercial distribution. These products remain experimental, and are only to be used for a limited amount of time during an ongoing emergency.
Regarding the consequences for the incorporation of pseudouridine in mRNA as a drug for therapeutic or vaccine purposes, Borchardt et al conclude that:
“Pseudouridine likely affects multiple facets of mRNA function, including reduced immune stimulation by several mechanisms, prolonged half-life of pseudouridine-containing RNA, as well as potentially deleterious effects of Ψ on translation fidelity and efficiency.”
Based on the currently available information, it appears to me that the extensive random incorporation of pseudouridine into the synthetic mRNA-like molecules used for the Pfizer/BioNTech and Moderna SARS-CoV-2 vaccines may well account for much or all of the observed immunosuppression, DNA virus reactivation, and remarkable persistence of the synthetic “mRNA” molecules observed in lymph node biopsy tissues (Roltgen et al. 2022). Many of these adverse effects were reported by Kariko, Weissman et al in their 2008 paper “Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability” (Kariko et al. 2008) and could have been anticipated by regulatory and toxicology professionals if they had bothered to consider these findings prior to allowing emergency use authorization and widespread (global) deployment of what is truly an immature and previously untested technology. Therefore, neither the FDA, NIH, CDC, nor BioNTech (which employs Dr. Kariko as a Vice President) nor Moderna can claim true ignorance. To my eyes, what we have seen is more appropriately classified as “willful ignorance”.
Based on my review of the scientific data, it is my opinion that the random and uncontrolled insertion of pseudouridine into the manufactured “mRNA”-like molecules creates a population of polymers which may resemble natural mRNA, but which have a variety of properties which are clinically relevant. These characteristics and activities may account for many of the unusual effects, unusual stability, and striking adverse events associated with this new class of vaccines. These molecules are not natural mRNA, and they do not behave like natural mRNA.
The question that most troubles and perplexes me at this point is why the biological consequences of these modifications and associated clinical adverse effects were not thoroughly investigated before widespread administration of random pseudouridine-incorporating “mRNA”-like molecules to a global population.
Biology, and particularly molecular biology, is highly complex and interrelated. Change one thing over here, and it is really hard to predict what might happen over there. That is why one must do rigorously controlled non-clinical and clinical research. Once again, it appears to me that the hubris of “elite” high status scientists, physicians and governmental “public health” bureaucrats has overcome common sense, well established regulatory norms have been disregarded, and patients have unnecessarily suffered as a consequence. These products do not use natural mRNA, and referring to them as mRNA vaccines is misleading. I recommend that, in the future, these products which employ a synthetic unnatural polymer which is not natural mRNA, should be designated using a different term, such as Ψ-mRNA genetic medicines.


Isn't this what getting the shot on the childhood schedule was all about? Now they'll be covered by the 1986 law, unless I'm misunderstanding (always a large possibility!)
I find this opening statement very hard to believe, particularly since the linked source is the incredibly non-credible New York Times. "Whatever you may currently think about the SARS-CoV-2 vaccines, it is a fact that more than 5.41 billion people worldwide have received a dose of some type of COVID-19 vaccine, equal to about 70.5 percent of the world population." What is true, is the vaccines that are not vaccines, are mRNA jabs that are not natural mRNA, were administered to a duped public for a virus that was created in a lab doing illegal gain-of-function research funded by Anthony Fauci.